Post-Award
The post-award phase occurs after an award has been received by the university. During this phase DCG will work directly with the sponsor, Principal Investigator, School, and any other necessary parties to negotiate terms in compliance with University Policy and execute the Award.
Post-Award Roles and Responsibilities
The University of Southern California is committed to helping its faculty successfully manage their sponsored research agreements. Doing this requires a partnership among faculty investigators, schools (and their departments, research centers and institutes), and central administration. The information below describes the roles and responsibilities of each of these groups as it relates to post-award actions.
Principal Investigator Responsibilities
Principal Investigators play a pivotal role in USC research projects, overseeing their direction, ensuring adherence to ethical standards, and fostering collaboration among team members. Their leadership and expertise are crucial for driving innovation, maintaining research integrity, and ultimately advancing knowledge in their field. Below is a list of common PI post-award responsibilities.
- Executing the project as outlined in the funded proposals.
- Carrying out the project’s financial plan as presented in the proposal.
- Making changes to the project’s financial plan following sponsor and/or USC’s policies and procedures.
- Reporting project progress and submitting deliverables to the sponsor as outlined in the terms of award.
- Responding promptly to staff requests for approvals, information, or decisions.
- Ensuring that an accurate record is maintained of project-related expenses.
- Selecting, training, and evaluating project staff and students.
- Complying with all USC policies and procedures, maintaining high standards of research integrity, and protecting the welfare of research subjects.
- Complying with all applicable sponsor rules, regulations and/or terms and conditions of the award.
Although the PI is usually assisted by administrative staff, the management of project funds and the ultimate responsibility for the financial and administrative management of the project rests with the PI.
Department/Center Research Administrator Responsibilities
Departmental Research Administrators are responsible for managing the administrative aspects of research projects, ensuring compliance with funding regulations, and facilitating smooth communication between researchers and funding agencies. Below is a list of departmental research administrator post-award responsibilities.
- Prepare budgets (including any satellite budgets), financial transactions (e.g., cost transfers, payroll, requisitions) and certify that cost sharing is documented.
- Review and approve financial transactions.
- Complete necessary adjustments to award charges and/or payroll
- Review the terms and conditions of the award to determine if any prior approvals are needed and notify DCG if /when prior approvals are requested.
- Prepare effort reports.
- Initiate supplier contracts and change orders in Workday and ensure all requested information is complete.
School Responsibilities
Schools are responsible for ensuring that administrative support is provided to investigators for the fiscal and administrative management of sponsored projects. In some schools, this support is provided centrally by the school as a service for all of the school’s PIs. In other schools, departments, centers, and/or institutes provide this service locally. It is the school’s responsibility to ensure that no PI is left without administrative support, that all PIs are aware of where to obtain support, and that sufficient resources are available to meet the needs of PIs. Below is a list of school- post-award responsibilities.
- Approve re-budgeting requests.
- Certify that cost sharing is documented.
- In the absence of the PI (e.g., transfer, long-term absence) prepare and submit technical reports to the sponsor and DCG.
- Approve PI changes.
- Approve PI Transfers.
Department of Contracts and Grants
The USC Department of Contracts and Grants is tasked with aiding Principal Investigators (PIs), Departments, and Schools in negotiating and executing agreements and amendments. They provide guidance on compliance with the terms outlined in sponsored agreements and facilitate the process of seeking sponsor prior approvals when necessary. Below is a list of DCG post-award responsibilities.
Award Acceptance and Set-Up
- Negotiate terms and conditions with sponsor.
- Confirm acceptance of exceptional terms and conditions.
- Approve award terms and conditions and if applicable, execute award/agreement.
- Confirm that regulatory compliance approvals are in place.
- Enter award information in Cayuse SP system.
Compliance
- Forward prior approval requests to the sponsor, when sponsor approval is required.
- Obtain sponsor’s decision regarding changes to the project and update university systems, as necessary.
- Assist PI/Dept Admins with questions regarding interpretation of terms.
- Assist with the non-financial closeout of the Agreement.
Outgoing Subawards
- Upon receipt of Workday, request, prepare, negotiate, and sign subcontracts/subawards and subsequent modifications.
Sponsored Projects Accounting
The Office of Sponsored Projects Accounting manages the post-award financial aspects of university-sponsored research, including the setup and modification of Award, Award Line, and Grant Worktag information. They handle detailed budget planning and reallocation, process invoices, manage accounts receivable, and generate financial reports. Additionally, they facilitate expense transfers to ensure accurate and compliant financial administration of sponsored projects. Below is a list of SPA post-award responsibilities.
All set up of awards in the Workday system.
Financial Management of Awards
Financial Reporting
Invoicing
Ensuring Payments
Rebudgets
Cost Transfers
For more information please visit SPA’s Website
Award Setup
Upon execution of an Agreement (or an amendment to an agreement), the award information is entered into the Cayuse SP system by DCG’s Award Establishment Team. Once the agreement is set up in Cayuse SP, a notification is set to SPA so that they can complete the set up in the Workday system. For additional information on how to check the status of your award set-up or review your award information in Cayuse SP, please visit our Cayuse SP Post-Award Resources page.
Required Trainings, Certifications and Regulatory Reviews
Prior to set up of the award or amendment in Cayuse there are a number of compliance checks DCG performs to ensure that all applicable trainings & certifications have been completed:
Trainings
Complete Grants Management Training:
Required for all USC personnel in a Principal Investigator (PI) or Co-Principal Investigator (Co-PI) role (as defined by the sponsor). Please confirm completion by looking up the PI in the “Admin” section of Cayuse SP and reviewing the section for “Training & Certifications.”
Complete Financial Conflict of Interest Training:
This training is required for all awards which have funding from a Public Health Service sponsor (e.g. DHHS, NIH, SAMSHA, HRSA) or Department of Energy for all personnel in an investigator role. “Investigator” means the project director or principal investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research. This must be completed every 4 years. PI and Dept Admins can confirm completion by checking in Cayuse SP under the “Admin” section and reviewing “Training & Certifications.”
Complete Research Security Training:
This training is required to be completed annually for all Department of Energy, National Institutes of Health and National Science Foundation awards by all covered individuals—those who play a substantive and meaningful role in developing or executing the project scope. At a minimum, this applies to all personnel designated as Senior/Key Personnel, including (but not limited to) the Principal Investigator/Project Director(s) and any Co-Principal Investigator/Project Director(s). Please confirm completion by looking up the PI in the “Admin” section of Cayuse SP and reviewing the section for “Training & Certifications.”
Certification and Regulatory Reviews
Conflict of Interest Review Committee (CIRC) Approval:
Required for all awards where the Cayuse indicates “Yes” to conflict of interest on the Regulatory Compliance Tab. Please request confirmation of Conflict of Interest Committee approval from PI/Dept Admin and provide it to your DCG officer.
Complete Medicare Coverage Analysis (MCA):
Required for all awards where there are patient care costs. Please work with the Clinical Trials Office and route through the Oncore System. Once the MCA is final and the Award/Amendment is final DCG will complete the Consistency Checklist in I-Star so approval of your study can be finalized in Oncore and you can begin to enroll patients.
Complete IRB Approval:
Required for all awards with human subjects. Please promptly provide this to your DCG Officer upon receipt. For additional guidance on how to request IRB approval please visit the IRB website. Please note that all IRB approvals will need to be connected with the Cayuse proposal or award Project # connected to the IRB approval. DCG has created a quick guide to assist: How to Link an IACUC/IRB Protocol to a Cayuse SP Proposal or Award.
Complete IACUC Approval:
Required for all awards where animal subjects are included. Please promptly provide this to your DCG Officer upon receipt. For additional guidance on how to request IACUC approval, please visit the IACUC website. Please note that all IACUC approvals will need to be connected with the Cayuse proposal or award Project # connected to the IACUC approval. DCG has created a quick guide to assist: How to Link an IACUC/IRB Protocol to a Cayuse SP Proposal or Award.
Complete Disclose:
This certification is required annually for awards which have funding from a Public Health Service sponsor (e.g. DHHS, NIH, SAMSHA, HRSA) or Department of Energy for all personnel in an investigator role and is required to be updated whenever a party needs to disclose a conflict of interest consistent with USC’s Conflict of Interest Policy. “Investigator” means the project director or principal investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research. PI and Dept Admins can confirm completion by checking in Cayuse SP under the “Admin” section and reviewing “Training & Certifications.”
Complete IP-ACT :
The National Institute of Standards and Technology requires that employees supported by federal grants and contracts provide a written present assignment of their grant/contract produced Intellectual Property (IP) to their institution, along with an agreement in writing to promptly disclose their IP. Employees meet this requirement through a one-time response to an email invitation sent from the “IP-ACT” system at USC. If you are a named investigator, and/or budgeted to receive income and/or paid from a federally funded project, you will be invited via email to log into the “IP-ACT” system and accept USC’s IP assignment and disclosure terms. Once you accept, you have satisfied the requirement for all future federal grants and contracts. Acceptance is required for all named project personnel prior to establishment or modification of federal awards. In addition, students and others who were not named in the award must complete these steps prior to being set up on payroll. Please confirm completion by looking up the PI in the “Admin” section of Cayuse SP and reviewing the section for “Training & Certifications.”
How to Register With Clinicaltrials.gov
On September 16th, 2016, The The U.S. Department of Health and Human Services issued a final rule that sets forth expanded requirements for registration and results information to clinicaltrials.gov for FDA-regulated drug, biological, and device products. Simultaneously, NIH issued a complementary policy requiring registration and results information to clinicaltrials.gov for all NIH-sponsored clinical trials, regardless of whether the trial is covered by the HHS Final Rule.
USC’s IRB’s Policy further states:
Registration and results reporting are required for applicable clinical trials; however, ClinicalTrials.gov allows voluntary reporting of other studies that:
- Are in conformance with any applicable human subject or ethics review regulations (or equivalent) and
- Are in conformance with any applicable regulations of the national (or regional) health authority (or equivalent) Investigators may choose to register a study that is not an applicable clinical trial as a condition to publish study results in a journal. FDA regulations require reporting of results from registered trials.
The Responsible Party must generally report results no later than 12 months after the trial completion date. Results must include participant baseline characteristics, participant flow diagram, outcomes, and adverse events. Instructions for submitting results are available at ClinicalTrials.gov. FDA also requires sponsors or investigators to certify compliance with ClinicalTrials.gov registration when submitting certain applications to the FDA. Form FDA 3674 is used to certify compliance.
The text above may be used in NIH applications requiring a statement of USC policy.
Registration is required by law and USC IRB policy for:
- FDA Clinical Trials defined as intervention studies that include drugs, biological products, and medical devices;
- NIH Clinical Trials defined as biomedical and behavioral studies of human subjects;
- Publication of research studies that assigns human subjects to health-related interventions and evaluates their outcomes. Other public registries are also available to meet criteria for ICMJE and WHO; and
- Reimbursement of Medicare claims for items and services in clinical trials that are qualified for coverage.
Registration of an applicable clinical trial must be submitted no later than 21 days after enrollment of the first participant, and the data that must be reported includes
- Participant Flow
- Demographic and Baseline Characteristics
- Primary and Secondary Outcomes
- Results of any Scientifically Appropriate Statistical Tests
- Adverse Event Information
Non-compliance of these requirements may result in civil monetary penalties, withholding/recovery of federal funds, and/or withholding of publications.
Principal Investigators are responsible to register their studies prior to participant enrollment in the Protocol Registration System (PRS).
Contacts to Request a PRS User Account
For Cancer Center Studies
- Tomi Homesy
- Email: Tali.Homsey@med.usc.edu
- Kathya Perez
- Email: Kathya.Perez@med.usc.edu
For Non-Cancer Studies & All Other Requests
- Kimberlee Eudy
- Email: eudy@usc.edu
- Phone: (858) 964-0730
Additional Information
Training Resources
- Final Rule Webinar Series (Overview of the Final Rule & Information Requirements)
- How to Submit Results Data (Overview of Each Results Module)
- Example Studies
STEPS TO REGISTER STUDY ON CLINICALTRIALS.GOV

Special Requirements for Federal Awards
Awards with funding from Dept of Energy/National Nuclear Security Administration
Conflict of Interest
The DOE regulation 42 CFR Part 50 Subpart F, Promoting Objectivity in Research (FCOI regulation), establishes standards that provide a reasonable expectation that the design, conduct, or reporting of Department of Energy funded research will be free from bias resulting from any Investigator’s conflicting financial interest. DOE requires recipient institutions and their investigators to fully comply with all FCOI requirements.
For more information about this policy please feel free to review:
In order to comply with FCOI requirements the following must be completed:
Complete Disclose:
Required for all personnel in an investigator role. “Investigator” means the Project Director or Principal Investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research. This must be completed annually. DCG can confirm completion by looking up the investigator in the “Admin” section of Cayuse SP and reviewing the “Training & Certifications” section.
Complete Conflict of Interest Training:
This training is required for all personnel in an investigator role. “Investigator” means the Project Director or Principal Investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research. This must be completed every 4 years.
Research Security Training
The U.S. Department of Energy (DOE) and the National Nuclear Security Administration (NNSA) have issued a new mandatory research security training requirement that applies to all active DOE awards and new proposals submitted to DOE on or after May 1, 2025.
Who Must Complete the Training:
Individuals currently working on DOE or NNSA funded projects who contribute in a substantive and meaningful way to the development or execution of the project scope are considered “covered individuals” and are required to complete the training. At a minimum, this includes the Principal Investigator/Project Director(s) and any Co-Principal Investigator/Project Director(s).
When Training is Required:
- New Proposals: Training must be completed prior to proposal submission after May 1, 2025.
- Active Awards: Training must be completed by May 1, 2025, and annually thereafter for the duration of the project.
How to Complete the Training:
To fulfill this requirement the Office of Ethics and Compliance (OEC) has added a 70-minute course entitled “Research Security Training” that is now available through TrojanLearn. OCEC will be reaching out via e-mail and assigning this course to all current covered individuals on active DOE awards so that they can more easily complete the training.
How Training Will be Verified:
- New Proposals: DCG will verify that all USC personnel named as “Senior/Key Personnel” in Section A of the Research & Related Budget formhave completed the required training and that their training is current. Proposal submissions will not proceed until the training is complete.
- Active Awards: DCG will verify that all USC personnel named as “Senior/Key Personnel” in Section A of the Research & Related Budget form have completed the required training and that their training is current. New award actions will be held until the training is complete.
- Current Awards: PIs, with the support of their department and school research administration, are responsible for ensuring any new covered individuals assigned to DOE or NNSA projects complete the training prior to working on the project.
For questions, please contact the Office of Ethics and Compliance at compliance@usc.edu
Requirements for Awards with Federal Funding
Complete IP-ACT:
Required for all personnel on federally funded agreement in order to comply with the Bayh-Dole Act. For additional information on this requirement, please review Bayh-Dole Act Obligations for Universities. PI and Dept Admins can confirm completion by checking in Cayuse SP under the “Admin” section and reviewing “Training & Certifications.” In the event that this has not been completed, please contact your DCG Officer and they will generate an e-mail to the faculty/staff member so that they can complete.
Awards with Funding from Public Health Services (PHS) or Department of Energy Sponsor
The HHS regulation 42 CFR Part 50 Subpart F, Promoting Objectivity in Research (FCOI regulation), establishes standards that provide a reasonable expectation that the design, conduct, or reporting of Public Health Services Sponsors (e.g. DHHS, NIH, SAMSHA, HRASA) or Department of Energy funded research will be free from bias resulting from any Investigator’s conflicting financial interest. HHS requires recipient institutions and their investigators to fully comply with all FCOI requirements.
For more information about this policy please feel free to review:
- NIH Financial Conflict of Interest – https://grants.nih.gov/grants/policy/coi/index.htm
- 42 CFR Part 50: https://www.govinfo.gov/content/pkg/FR-2011-08-25/pdf/2011-21633.pdf
In order to comply with FCOI requirements the following must be completed:
Complete Disclose:
Required for all personnel in an investigator role. “Investigator” means the Project Director or Principal Investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research. This must be completed annually. DCG can confirm completion by looking up the investigator in the “Admin” section of Cayuse SP and reviewing the “Training & Certifications” section.
Complete Conflict of Interest Training:
This training is required for all personnel in an investigator role. “Investigator” means the Project Director or Principal Investigator and any other person, regardless of title or position, who is responsible for the design, conduct, or reporting of research. This must be completed every 4 years.
Post-Award Guidance
The information below will aid you in developing a deeper understanding of cost principles and various prior approval scenarios to guarantee that the award remains on course and aligns with the stipulations set forth by the funding organization.
Be Familiar With Award Terms and Conditions
In order to effectively manage an Award it is important to carefully review the terms and conditions of the agreement which are accessible in Cayuse SP. To access your award in Cayuse please visit our Cayuse SP Post-Award Resources page which offers a set of customized web guides that aim to walk users through understanding how to look up Award records and retrieving Award data (including notice of award documentation) directly within the Cayuse SP system.
In order to effectively manage an Award, it is important to carefully review the terms and conditions of the agreement which are accessible in Cayuse SP.
Understanding and Applying Cost Principles
Federal guidance (as outlined in 2 CFR 200) lays out cost principles that should be applied to all awards to ensure compliance and transparency in financial management. These principles dictate that all costs charged to federally funded projects must be allowable, allocable, reasonable, and consistent.
“Allowable” means the costs meet specific criteria outlined by regulations or the terms of the award.
“Allocable” signifies that costs are directly associated with the project and can be assigned to it in a reasonable manner.
“Reasonable” denotes that costs do not exceed what a prudent person would incur under similar circumstances.
“Consistent” indicates that costs are treated uniformly across projects and in accordance with institutional policies and procedures.
Adhering to these principles helps ensure responsible stewardship of federal funds and promotes accountability in research expenditures.
Allowable
A cost is allowable if it is permitted as a cost within general award regulations, the terms of a specific Award, and/or USC’s F&A rates.
To be allowable, a cost must comply with the below standards:
Be necessary and reasonable for the performance of the award and be allocable thereto under these principles.
Conform to any limitations or exclusions set forth in the agreement as to types or amount of cost items.
Be determined in accordance with generally accepted accounting principles (GAAP).
Not be included as a cost or used to meet cost sharing or matching requirements of any other federally financed program in either the current or a prior period.
Be adequately documented.
Be incurred during the approved budget period.
Costs expressly unallowable or mutually agreed to be unallowable should be identified and excluded from any billing, claim, application, or proposal related to the Sponsored Award. Inclusion of an unallowable cost in a proposal does not make the cost allowable. Adding a justification to an unallowable cost in a proposal also does not make the cost allowable and sponsor explicit written approval will be required.
Allocable
A cost is allocable to a particular Award if the goods or services involved can be directly allocated to the Award it is being charged to without undue difficulty.
To determine if a cost is allocable, ask the following questions:
- Is the cost incurred specifically for this award?
- Can it be easily justified as allocable to this award without undue difficulty and with a high degree of accuracy?
If a cost benefits two or more projects or activities in proportions that can be determined without undue effort or cost, the cost must be allocated to the projects based on the proportional benefit. If a cost is not allocable then it cannot be directly charged to a sponsored agreement could be considered an indirect cost and so should not be charged as a direct cost.
Reasonable
A cost is considered reasonable if the nature of the goods or services, and the price paid for the goods or services, reflects the action that a prudent person would have taken given the prevailing circumstances at the time the decision to incur the cost was made.
To determine if a cost is reasonable, ask the following questions:
- Is the cost necessary for the performance of the Sponsored Award?
- Does incurring this cost violate the restraints or requirements imposed by federal and state laws and regulations, or Sponsored Award terms and conditions?
- Is the price of the goods or services comparable from multiple vendors/sources that have no vested interest or relationship to the Award or to the person involved in the purchase?
- Have the individuals incurring this cost acted with due prudence (discretion and good sense) in the circumstances? Have they considered their responsibilities to the institution, its employees and students, the federal government, and the public at large?
- Were the actions that were taken in respect to incurring the cost consistent with established institutional policies and practices applicable to Sponsored Awards?
Consistently Treated
All costs incurred for the same purpose and in like circumstances must be treated uniformly either as direct costs or as indirect (facilities and administrative or F&A) costs. All costs must be treated consistent with University policies and procedures that apply uniformly to both federally financed and other activities of the non-Federal entity.
Common Post-Award Actions
This section offers a comprehensive overview of post-award actions that might come up during the life of the Award. Whether you need to modify your budget, transfer funds between accounts, or make personnel changes to your project, this guide facilitates seamless navigation through these post-award protocols.
Carryover/Carryforward of Funds
Carryover is a process through which unobligated funds remaining at the end of the budget period may be carried forward to the next budget period. The carryover of funds allows a USC PI to use the unused prior year funds in the current budget period.
The award document will contain clear guidance specifying if carryover of funds between budget periods is allowed or requires sponsor prior approval. If prior approval of carryover is required by the sponsor and the PI wishes to carryover funds to the new budget period, please promptly notify your DCG Officer so that they can work with the sponsor to get approval.
Documentation required by the sponsor to request carryover approval may vary and so you will want to review the award and work with your DCG Officer to determine what is required.
Generally, the following documents are required for carryover requests:
A programmatic justification for the requested carryover.
The amount of funds requested to be carried over from the current period to the new period.
A budget and justification for the carryover funding.
Once the required documents have been provided, your DCG Officer will reach out to the sponsor to request approval. When approval is received, your DCG Officer will request that the award amount for the current period is updated in Cayuse SP.
Change in Scope
All sponsors will require prior approval for changes to the approved scope of work. Documentation required by the sponsor to request approval may vary and so you will want to review the award and work with your DCG Officer to determine what is required.
Generally, the following documents are required to request a change in scope:
- Justification for Requested Changes to the Scope of Work
- A Revised Scope of Work
- A Revised Budget and Budget Justification
- If this change includes change in cost share amounts, change in F&A rate, change in PI, or an increase in the award amount, a revised Cayuse SP record might be needed.
Once you have provided the required documents, your DCG Officer will reach out to the sponsor to request approval. When approval is received, your DCG Officer will request that the award be updated in Cayuse SP.
Budget Changes/Rebudgets
The budget is the financial plan for the project or program that the sponsor approves during the proposal and award process. During the course of the award, revisions to the budget might be necessary. Rebudgeting requests not requiring sponsor prior approval should be sent directly to Sponsored Projects Accounting for processing. Rebudget requests requiring sponsor prior approval should be directed to your DCG Officer to secure sponsor prior approval prior to requesting Sponsored Projects Accounting modify the Award budget in Workday.
Generally, the following documentation are required for rebudget approvals:
A programmatic justification for the requested rebudget.
A revised budget and justification using the template required by the sponsor.
Confirmation that this does not represent a change in the Scope of Work.
If this change includes altering cost share amounts, change in F&A rate, a change in PI, or increase in the award amount, a revised Cayuse SP record might be needed.
Upon receipt of the documentation required by the sponsor, your DCG Officer will review and let you know if any additional documentation or action is required. DCG will request approval of the rebudget. Once approval is received, you can work with Sponsored Projects Accounting to have the budget modified in the financial system.
Here are some common rebudget issues you might encounter that might require prior approvals:
Award Reduction
Occasionally a sponsor will request to reduce the amount of funds that have already been committed to a sponsored project. Upon receipt of such a notification or amendment from the sponsor, please notify your DCG Officer and provide the following information:
The documentation or request from the Sponsor and any related communications with the sponsor (e.g., revised budgets, revised scopes).
Confirmation from the PI that this reduction is either acceptable or not acceptable.
Confirmation from the PI that the scope of work either has or has not been revised or reduced.
Upon receipt of the above, your DCG Officer will work with you and the sponsor to resolve and finalize this change to the award and have the award updated in the Cayuse SP system.
Rebudgeting - Add Equipment
Sponsor requirements regarding the allowable purchase of equipment funded with grant and contract budgets are dependent upon individual sponsor regulations and/or type of award. In the event equipment was not budgeted at proposal stage and now needs to be purchased, it is common for sponsor’s to require prior approval for this change.
Normally the sponsor will request the following information:
Description/Quote for the Equipment
Revised Budget
Justification for the Purchase
Purchase of Equipment in Last 3 Months of a Project
The purchase of equipment in the last three months of a project requires prior University approval even if the sponsor-approved budget included funds for the equipment. Extraordinary justification will be necessary for such purchases, except in cases where a renewal award is imminent.
All requests must include:
A description of the equipment.
An estimate of the cost.
An indication of whether the equipment is in the sponsor-approved budget.
A description of why the equipment is necessary to complete the project.
All requests must be approved by:
- The Principal Investigator
- Chair/Unit Head or Designee
Please provide approval to Sponsored Projects Accounting once received.
Rebudgeting - Add Subaward
Often, the addition of a subaward to the budget will require prior approval. If prior approval is required, you will need to notify your DCG Officer. Documentation required by the sponsor to request approval may vary and so you will want to review the award and work with your DCG Officer to determine what is required.
Generally, the following documents are required in order to add a Subaward(s):
- Justification for the requested addition.
- Subrecipient Scope of Work, Budget/Budget Justification and Subaward Certification Form or Letter of Commitment.
- Revised USC Budget (incorporating the Subaward).
- Confirmation that the Subaward(s) either do or do not represent a change in the Scope of Work.
Significant Rebudgets - Possible Change in Scope
Significant award rebudgets could indicate a change in the Scope of Work, which would require sponsor approval. Please note that University Policy requires any cumulative rebudget of greater than 25% of the sponsor approved budget to be reviewed by DCG for possible change in scope. DCG requires confirmation from the PI that there has been no change in scope or, if this does represent a change in scope, will assist the PI with getting approval.
All sponsors will require prior approval for changes to the approved scope of work. Documentation required by the sponsor to request approval may vary and so you will want to review the award and work with your DCG Officer to determine what is required.
Generally, the following documents are required to request a change in scope:
- Justification for Requested Changes to the Scope of Work
- A Revised Scope of Work
- A Revised Budget and Budget Justification
- If this change includes change in cost share amounts, change in F&A rate, change in PI, or an increase in the award amount, a revised Cayuse SP record might be needed.
Once you have provided the required documents, your DCG Officer will reach out to the sponsor to request approval. When approval is received, your DCG Officer will request that the award be updated in Cayuse SP.
Changes in Key Personnel
Key personnel changes can significantly impact the trajectory and execution of an award and often require sponsor prior approval. Managing these personnel changes effectively requires clear communication and proactive planning to ensure project continuity and success. Below are common changes to key personnel that you might encounter:
Change of Principal Investigator (PI)
Sometimes a Principal Investigator on an Award can no longer serve as PI or is leaving the University. If the school decides that it is in the best interest of the scope of work for the award to remain at USC, they can request that a new faculty member be appointed to assume the role of PI.
In the event that the school would like to request a new PI be added or appointed to an Award, they should promptly notify their DCG Officer and process a revised Cayuse SP record, adding the new PI to the award and securing PI and school approvals.
Generally, the following documents are required for these requests:
- If the PI is leaving USC, an Outbound Assessment.
- A Justification for the Requested Change.
- The CV and/or Biosketch and/or Other Support for the New PI.
- A Revised Scope of Work (if applicable).
- A Revised Budget/Budget Justification (if applicable).
Once you have provided the required documents, your DCG Officer will reach out to the sponsor to request approval. When approval is received, your DCG Officer will update the PI information in Cayuse SP.
Changes in Senior/Key Personnel
Who are Senior/Key Personnel?
Senior/Key Personnel are individuals named on a sponsored proposal under the “Senior/Key Personnel” sections and who are essential to the project’s successful execution. While this includes the Principal Investigator (PI), it also includes USC personnel in other critical roles such as Co-Investigators, and any other individuals named as senior/key Personnel.
When Changes Occur
If a member of the senior/key personnel can no longer serve or leaves the University, federal sponsors (and frequently other sponsors) require prompt notification and prior approval.
What to Do
If changes occur, the PI and/or Department should promptly notify their DCG Officer and provide:
- A justification for the requested change
- CV, Biosketch, and/or Other Support for any new senior/key personnel
- Confirmation of whether the change will affect the project’s scope of work
- Any additional documentation required by the sponsor
Your DCG Officer will review the request and reach out if additional information is needed. DCG will then contact the sponsor to request approval. Once approval is received, your DCG Officer will provide written confirmation to the PI and Department for their records. Any changes in senior/key personnel should also be documented in progress reports.
Disengagement of PI of 90 Days or More
Sponsors reasonably expect that the PI will be physically present at the location where their funded project is carried out to manage the award and ensure that objectives and timelines are met. Most sponsors want to be notified when a PI will be disengaged for periods of time exceeding ninety days. Federal sponsors in particular require advance notification when a PI will be disengaged for a period of time extending 90 days or more (e.g., a medical leave). Individual awards should be reviewed for specific guidance. In the event that it becomes necessary to appoint an interim PI, please promptly notify your DCG Officer.
Generally, the following documents are required for these requests:
- A justification for the appointment of an interim PI, including the interim PI’s name and expected period of time for appointment.
- CV or Biosketch for the interim PI.
- A revised Cayuse SP record collecting school and PI approvals and certifications for the interim PI.
Once the required documents have been provided to DCG, your DCG Officer will reach out to the sponsor to request approval. When approval is received your DCG Officer will update the PI information in Cayuse SP.
Principal Investigator’s Departures
When a faculty member intends to leave USC, please promptly inform your DCG Officer and complete the Outbound Assessment form for all active proposals and awards for the departing PI.
The University will need to promptly inform the sponsor and engage in a process by which the University seeks sponsor approval to transfer active awards to the PI’s new institution, appoint a new USC PI, or terminate the project early.
For awards that will be transferred to the departing PI’s new institution, please also complete the Award Transfer Checklist to ensure all items are complete and USC has met all of its obligations prior to relinquishing to the new institution.
Reduction of PI Effort
Sponsors may require that the PI maintain a certain percentage of effort and/or require sponsor prior approval regarding changes to PI or Key Personnel effort. If a reduction in effort requires prior approval, please promptly notify your DCG Officer.
Documentation required by the sponsor to request revisions to effort may vary and so you will want to review the award and work with your DCG Officer to determine what is required.
Generally, the following documents are required for requests:
- Revised percentage of effort requested for the PI and/or Key Personnel.
- A revised budget and budget justification with updated effort.
- Confirmation that this does or does not represent a change in approved scope.
Please Note: Any faculty in a PI role on an award must have some percentage of effort as they are responsible for the oversight and programmatic conduct of the scope of work under the award. This applies to all awards with the exception of equipment grants, fellowship awards, and awards where the sponsor does not allow PI salary. In the event PI effort is not charged directly to the award, this would be considered cost share and should be documented in Cayuse SP for school approval at the proposal stage.
Transfer of PI to New Lead Unit within USC
If the PI of an active award is transferring to a new lead unit within USC and wishes to have the management of the award moved to the new school/department, please notify your DCG Officer and provide the below documentation and approval:
Documentation Required:
- A list of awards to be transfers (please include sponsor and sponsor award number).
- The new department name and ID number.
- The effective date of the requested transfer.
- Contact information of the primary Research Administrator in both the current and new department.
Approvals Required:
- Principal Investigator.
- Chairs/Unit Heads or designees of current and new departments/units.
- Deans/Directors or designees of current and new schools/colleges, if also transferring to a new school.
To streamline the process, below is a helpful form to collect the information and approval. Once completed and all approvals are in place, please provide it to your DCG Officer to update your awards:
Early Termination
In certain instances, a project may be terminated prior to the original expiration date.
Possible reasons for early termination include:
- The PI has expended all of the awarded funding in compliance with the award terms and conditions and has submitted all project deliverables.
- The PI is leaving USC, and the project will not be transferred or assigned a new PI.
- The Sponsor has requested an early termination.
Award agreements typically include a clause for how an early termination situation will be handled, so individual awards should be reviewed for specific guidance. USC does not terminate awards early without very good justification. If a PI requests early termination, this should be directed to your DCG Officer for review to determine if the justification is sufficient and there is no other way to resolve the issue before moving forward. This request may require additional levels of review and the involvement of the school and the Office of General Counsel.
Generally, sponsor-initiated termination notices will be sent to DCG, in which case your DCG Officer will promptly inform the PI and School of the termination and have the award updated in Cayuse SP. Should you receive a notice of termination, please promptly provide it to your DCG Officer as swift action may need to be taken to stop work on the Award. The PI will need to work with the School and Sponsored Projects Accounting to ensure that all expenses are accounted for so that final invoices can be processed.
No-Cost Extension (NCE)
A no-cost extension is a prolongation of the project period beyond the original end date without additional funds from the funding agency. It allows the project team more time to complete the work and fulfill the project’s objectives within the existing budget. If additional time is needed, normally sponsor notification and/or approval is required. The department/PI should promptly inform DCG of the request and provide DCG with the following information:
- How much additional time is needed? What will the revised end date be?
- Justification for the need for additional time – the justification should include an allowable programmatic reason to request additional time. The justification needs to be related to the need to complete the scope of work and not to expend remaining funding.
- Any other documentation requested/required by the sponsor.
Please note, often, there is a limited time frame to request additional time and so it is important to promptly informing your DCG Officer.
Unobligated Balance Transfer
For firm fixed-price agreements, the PI may request to transfer unobligated funds to an unrestricted account after closeout of the Award. To request this, you will need to complete an Unobligated Balance Transfer Form and get the approval of the PI, Dept Chair, Dean and Sponsored Projects Accounting. This will then need to be forwarded to your DCG Officer for final review and approval.
The following criteria should be confirmed prior to submitting this form for approval:
- The Award qualifies as a Fixed-Price Agreement.
- All sponsor payments have been received by USC.
- All project expenses have been paid and encumbrances released.
- All milestones achieved, deliverables and reports delivered, and the Award Closeout process completed.
Once DCG approves the form, they will provide the signed Unobligated Balance Transfer Form to Sponsored Projects Accounting so that the amount can be transferred in the Workday financial system.
Outgoing Subawards
Principal Investigators may desire to enter into an agreement with a subrecipient to complete a portion of the proposed project. Please visit the Subawards page for more information.
Please note that the post-award process can involve various additional steps and activities depending on the specific requirements of the funding agency and USC’s internal procedures. This explanation provides a general overview, and it’s recommended to refer to USC’s official documentation or contact your DCG Officer for the most accurate information.
Award Closeout
Award Closeout is the process of finalizing all administrative and programmatic requirements for a sponsored award. This includes submitting all required reports, reconciling financial accounts, and returning any unused funds to the funding agency. Award Closeout typically begins within a few months of the end of the award period, but the specific timeline may vary depending on the funding agency and the type of award.
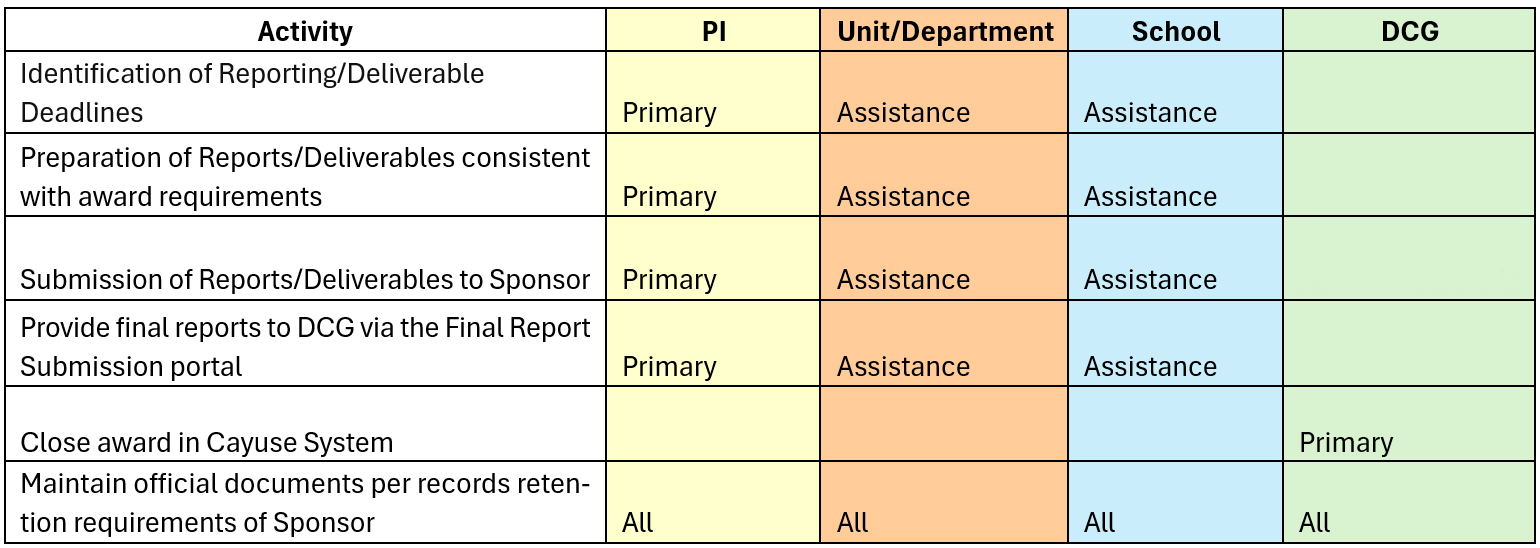
Roles and Responsibilities in the Award Close Out Process
Successful technical award closeout is a collaborative effort involving the Principal Investigator (PI), Unit/Department, School, and the Department of Contracts and Grants (DCG). The PI, with the assistance of their school, is primarily responsible for identifying awards with upcoming reports, submitting final reports to the sponsor, and providing copies to DCG Closeout Team via the Final Report Submission portal. DCG is responsible for uploading final reports to Cayuse and Laserfiche and closing the award in Cayuse. All parties share responsibility for maintaining official documents per sponsor retention requirements. The financial closeout, including final invoices, financial reporting and ensuring all costs are correctly incurred and payments are received is managed by Sponsored Projects Accounting.
Accessing Report Due Dates – Workday Grants Management Dashboard and Reports
The Cayuse Data tab in the Workday Grants Management Dashboards (for both Principal Investigators and Research Administrators) compiles data on active awards from Cayuse to provide details on the due dates for your final reports, based on the sponsor’s requirements and award terms. The dashboards are configured to display reports according to Workday security roles of Principal Investigators or Research Administrators.
Steps in Award Close Out
Review the award terms and conditions. This will help to ensure that all requirements have been met and that the award is in good standing.
Submit all required reports. This typically includes a final technical report and a final financial report. Some funding agencies may also require additional reports, such as a final invention statement or a human subject’s research closure report.
Reconcile financial accounts. This involves ensuring that all expenses have been properly charged to the award and that all unused funds are returned to the funding agency.
Close out all Subawards. If the award includes Subawards, these will need to be closed out in accordance with the funding agency’s policies and procedures.
Finalize all administrative tasks. This may include completing any outstanding paperwork, such as timesheets or travel reimbursement requests.
Once all of these steps have been completed, the award will be officially closed out. The funding agency will typically issue a notice of award closure, which will release the institution from any further obligations under the award.
Types of Final Reports
The specific final reports required for Award Closeout will vary depending on the funding agency and the type of award. However, some of the most common final reports required for Award Closeout include:
- Final Technical Report:The Final Technical Report summarizes the project’s accomplishments, findings, and conclusions. It also discusses the project’s impact on the field and any potential future research directions.
- Final Invention Statement: The Final Invention Statement discloses any inventions or discoveries that were made during the project. This information is used by the funding agency to determine whether any intellectual property rights need to be protected.
- Final Property Report:The Final Property Report accounts for all tangible property that was purchased or acquired with funding from the award.
- Final Subrecipient Reports:If the award included subawards, the institution will also need to submit final reports for each subaward. These reports will typically include the same information as the Final Technical Report, but for the subaward specifically.
In addition to these final reports, the institution may also be required to submit other documentation, such as data management plans, human subjects research closure reports, and animal welfare reports. The funding agency will provide specific instructions on the final reports and documentation that is required for Award Closeout.
Submission of Final Reports to the Sponsor
Final Reports are typically submitted to the sponsor electronically, through a web-based portal or email. Some sponsors may also accept Final Reports in paper form, but this is less common. To submit a Final Report electronically, the Principal Investigator (PI) will need to log into the sponsor’s web portal and upload the report file. The report file should be in a format that is compatible with the sponsor’s requirements, (such as PDF or Word).
Before submitting the Final Report, it is important to proofread it carefully to ensure that it is complete and accurate. The PI should also check with the sponsor to make sure that the report is in the correct format and that it has been submitted to the correct email address.
Below are some common Sponsor Award Closeout forms.
Providing Final Reports to DCG
All Final Technical Reports, Final Invention Statements, Final Property Reports, and Final Subrecipient Reports should be submitted to the Department of Contracts and Grants (DCG) via the Final Report Submission Portal. Once received, the Award Closeout Team in DCG will internally close out the award in the University’s data system (Cayuse SP).
Subaward Close Out
If the sponsored award included any Subawards, the closing out of each Subaward is also a pertinent factor in the overall closeout of the larger award. This often includes submitting all required reports, reconciling financial accounts, and returning any unused funds to the Prime Awardee (USC).
Here are some of the key steps involved in Subaward Closeout:
Review the Subaward Agreement. This will help to ensure that all requirements have been met and that the Subaward is in good standing.
Finalization of all Administrative Tasks. This may include completing any outstanding paperwork, such as timesheets or travel reimbursement requests.
Submission of all Required Reports. This typically includes a final technical report and a final financial report from the Subawardee.
Reconcile Financial Accounts. This involves ensuring that all expenses have been properly charged to the Subaward and that all unused funds are returned to the Prime Awardee (USC).
Once all of these steps have been completed, the subaward will be officially closed out. PIs are encouraged to remind subrecipients of these needs well in advance of the due date for such reports.
The Importance of Timely Submission of Final Reports
Final reports typically summarize the project’s accomplishments, expenditures, and other relevant information. Submitting these reports on time is an important part of the research administration process, as it allows funding agencies to assess the impact of their investments and ensure that funds are used appropriately.
What Final Reports Do:
- Demonstrate Accountability: Final reports allow funding agencies to see how their funds were used and how the research project contributed to the advancement of knowledge.
- Provide Feedback: Final reports provide feedback to funding agencies on the strengths and weaknesses of their research programs. This feedback can help funding agencies to improve their programs and make better decisions about how to allocate resources.
- Promote Collaboration: Final reports can be shared with other researchers and stakeholders, which can promote collaboration and new discoveries.
- Disseminate Research Findings: Final reports can be published and disseminated to the public, which helps to raise awareness of research findings and their potential impact on society.
Overall, final reporting is an important process that helps to ensure the integrity and quality of research. It also helps to promote accountability, collaboration, and the dissemination of research findings.
Timely submission of final reports on awards is critical to maintaining compliance with sponsor requirements and protecting future funding opportunities. Failure to submit reports on time can result in serious consequences, including suspension of funding for the PI or even the entire university. It may also lead to audit findings, financial penalties, and reputational damage. Ensuring timely and accurate submission helps maintain strong sponsor relationships, avoids administrative issues, and supports continued research funding.
Questions?
If you have questions regarding the Final Reporting requirements of a sponsored project, please contact DCG’s uscaward@usc.edu.